Knollwood has been a leader during COVID-19 as our leadership has worked tirelessly to engage its crisis preparedness plan and find new solutions to mitigate the coronavirus in our community.
The leadership is hyper-focused on protecting each area of Knollwood, which includes independent living, assisted living, and skilled nursing areas. These “neighborhoods” as we call them, are unique, and we were proud the independent living residents remain COVID-free.
As way of additional background, in early March when COVID-19 was first impacting the eastern part of the U.S., our management team set up a Clinical Review Board. The review board’s role is to swiftly address any critical health issues. The same is true for a daily directors’ call with up to 20 Knollwood managers who review their department’s status and immediate needs. Across every part of Knollwood, the professionals running the community are fully informed and collaborate to address the challenges presented by this pandemic.
Then, the leadership ensures those updates are shared with residents, staff, and family members. The ongoing written and verbal communications include Centers for Disease Control guidance and the detailed actions being taken at Knollwood to support the community’s understanding and ability to work together to contain and mitigate the virus.
It was in early April, that Knollwood’s clinical review board realized it was essential that all staff and residents – especially those without symptoms – had to be tested for COVID-19 to contain the virus. Public health authorities were not testing asymptomatic carriers and Knollwood’s leadership knew the asymptomatic had to be identified. Knollwood needed to find out if there were any asymptomatic carriers among the residents and staff. Knollwood’s CEO Tim McHale and COO Paul Bricker required help and reached out to the Department of Health to ask if they’d test everyone and were told they could not help. This was the breaking point. Leadership sprang into action and found a private lab to start testing all Knollwood staff and residents within 48 hours.
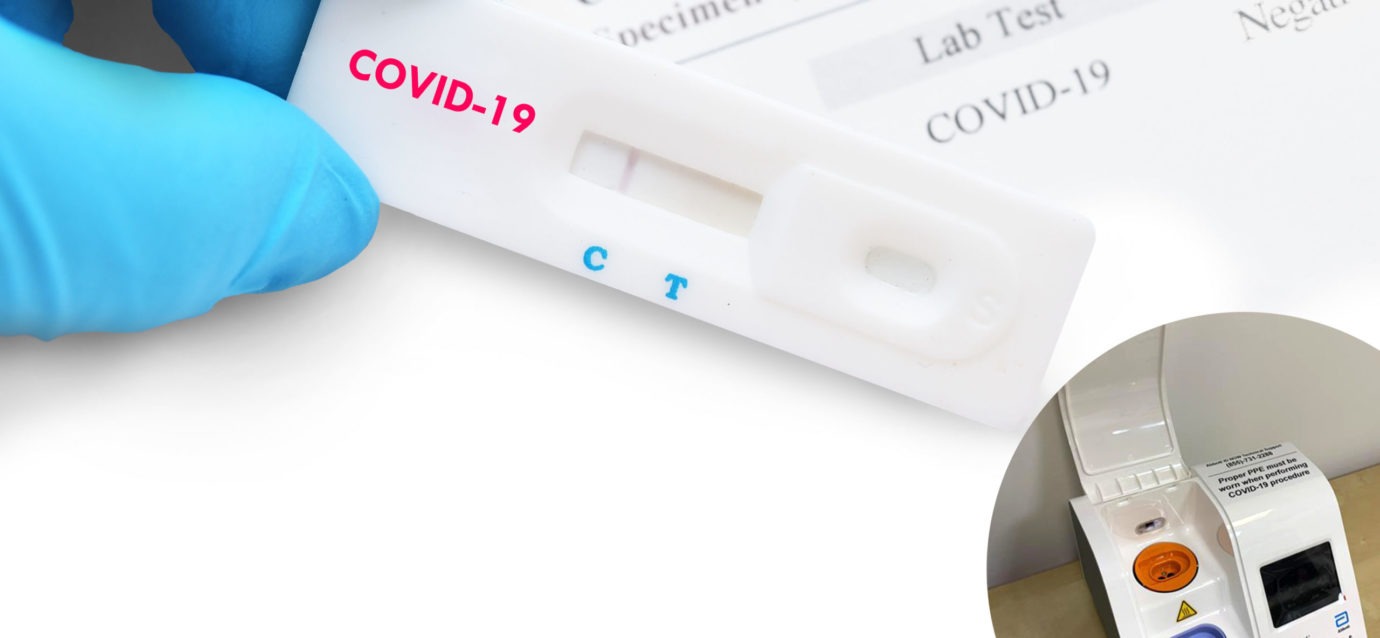
Concurrently, a local donor heard the news and was able to acquire and donate an Abbott Rapid ID Now® COVID-19 testing machine to Knollwood so its clinical team could conduct onsite, real-time tests.
“The donation of the Rapid ID Now® equipment has been essential to further assuring residents and staff that anyone who may not feel well is identified and tested immediately so they can receive medical attention reducing the probability of impacting the health of others at Knollwood,” said Margo Buda, R.N., Independent Living Clinic Nurse Manager. “It has been a significant tool in our fight against this invisible virus. We are blessed a donor made this happen so Knollwood has one more layer of protection.”
The Rapid ID Now® test machine processes COVID-19 tests administered by certified clinicians who have undergone extensive medical training by health regulators. All test results are also verified by an off-site lab. The lab ensures Knollwood is ready to process tests any time to remain as safe as possible until a vaccine is developed.